When Did Pfizer Get Approved
Food and Drug Administration approved the Pfizer-BioNTech coronavirus vaccine on Monday for people 16 and older making it the first of three COVID-19 shots available in the US. 1 day agoA decision had been expected by early 2022 after US.
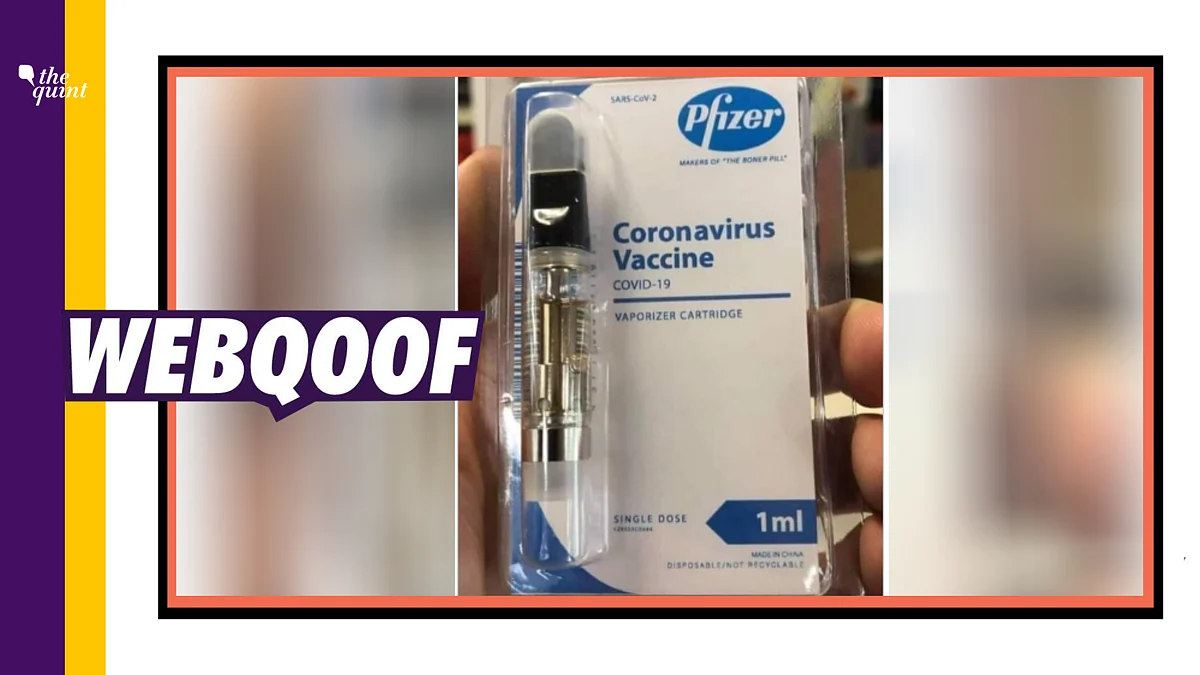
Fact Check Coronavirus Vaccine Image Shared As Made In China Pfizer Covid 19 Vaccine Is A Hoax
BNTX announced today that the US.
When did pfizer get approved. This does not affect the length of the clinical trial period. Has reaffirmed its findings that the Pfizer Covid-19 vaccine is safe and effective and the FDA. 11 2020 the Pfizer-BioNTech COVID-19 Vaccine has been available under EUA in individuals 16 years of age and older and the authorization.
Bukaty File LAURAN NEERGAARD and. The decision first reported by The New York Times marks a historic scientific achievement and suggests an end to the coronavirus pandemic may be in sight although. The Food and Drug Administration approved the Pfizer-BioNTech COVID-19 vaccine for 12- to 15-year-olds in the US.
So with the FDA fully approving the Pfizer vaccine do you think were gonna see more businesses in this area mandate the vaccines for their employees. FDA said to fast-track Pfizer shot full approval by The New York Times August 4 2021 at 353 am. Pfizers shot was first authorized as part of emergency rules back in December 2020.
The first to be authorized for children under age 16. Pfizers COVID-19 vaccine got emergency authorization from federal regulators on Friday clearing the way for the shipment distribution and eventual administration of the shots. On Monday the FDA amended its previous emergency use authorization for the Pfizer vaccine which only allowed inoculation of those 16 and up to include the younger age group.
PFE and BioNTech SE Nasdaq. Lets talk just for a minute about what this means for us as a healthcare systemWe have continually been urging our employees to get vaccinated we care deeply about our patients our employees and our community. The FDA granted it Priority Review meaning FDA officials planned to take action within six months of its release.
In this case they said they pulled in extra help and worked around the. The decision would apply to people 16 and older. FDA approval of Pfizer Covid shot could come next week.
Gave full approval to Pfizers COVID-19 vaccine on Monday Aug. Regulators agreed to a priority review of Pfizers application in July though many were. Has given its full and final approval.
Pfizer and BioNTech expect to file a Biologics License Application for possible full regulatory approval in 2021 NEW YORK MAINZ Germany--BUSINESS WIRE-- Pfizer Inc. Some people have been hesitant to get the COVID-19 vaccine until it was approved but they dont have to wait any longer. So just how did Pfizers COVID-19 vaccine for those 16 and older get approval so quickly.
Food and Drug Administration FDA has authorized the emergency use of the mRNA vaccine BNT162b2 against COVID-19 in. 13 hours agoWhile the Pfizer vaccine was approved under emergency use in December of 2020 Rai said the first studies started all the way back in March of. 1 day agoAfter a strict process the FDA.
Officials hope it will convince some vaccine holdouts to get. Food and Drug Administration issued the first emergency use authorization EUA for a vaccine for the prevention of coronavirus disease 2019 COVID-19. The bottom line.
December 14 2020 -- The CDC signed off on the Pfizer-BioNTech COVID-19 vaccine the agency announced on Sunday afternoon completing the final approval for. Pfizers shot is the first COVID-19 vaccine to gain full FDA approval. The Pfizer COVID-19 vaccines full approval has been long-awaited and many are hoping that it will help others feel more comfortable receiving it.
1 day agoSince Dec. 1 day agoThe FDA formally approved the two-dose Pfizer-BioNTech COVID-19 vaccine for people 16 and over on Monday opening the door for more vaccine mandates across the country. Previously the regulatory agency had allowed the shots to be administered under an emergency use authorization a mechanism used during public.
Mass General Brigham which employs 80000 workers in Massachusetts said it will require employees to get vaccinated once the FDA issues its full approval for at least one of the vaccines as did.

Covid 19 Eu Seals Deal For Extra 1 8 Billion Biontech Pfizer Shots

Pfizer Vaccine Looks Promising But Logistical Challenges Lie Ahead France 24

Covid Vaccine Which Covid 19 Vaccine Is Better Comparing Vaccines Astrazeneca Vs Moderna Pfizer And Johnson Johnson Marca

Fda Vows All Hands On Deck Effort To Get Pfizer Coronavirus Vaccine Full Approval As Quickly As Possible The Washington Post
No comments