Pfizer Emergency Approval
1 day agoPfizers shot will continue to be dispensed to 12- to 15-year-olds under an emergency use authorization until the company files its application for full approval. 1 day agoThe FDA like regulators in Europe and much of the world initially allowed emergency use of Pfizers vaccine based on a study that tracked 44000 people 16 and older for at least two months.
/cloudfront-us-east-1.images.arcpublishing.com/pmn/Q6RLXGI4G5G3LCOIUD6PKL4P2E.jpg)
Johnson Johnson Asks For Approval Of Its One Shot Vaccine Pa Lawmakers Beyond Frustrated With Vaccine Rollout Cases And Deaths Trending Down
Even after hundreds of millions of shots serious side effects such as chest pain and heart inflammation in teens and.
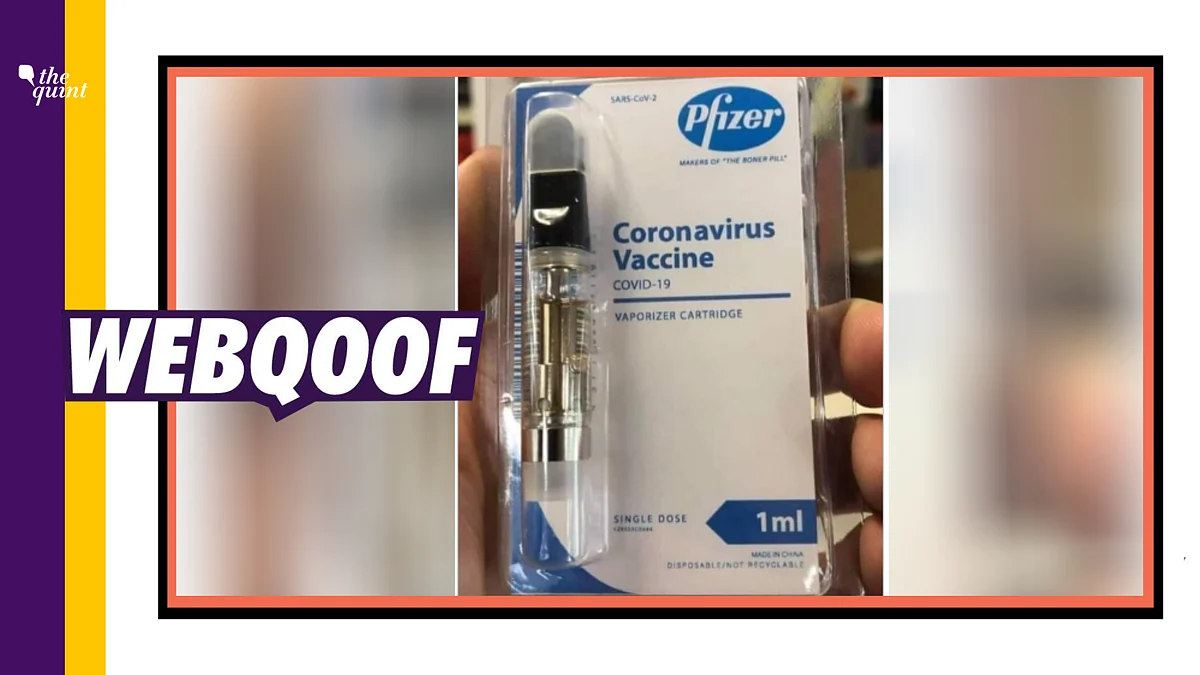
Pfizer emergency approval. Is the Pfizer vaccine FDA-approved. The first EUA approval. 1 day agoPfizers shot still has emergency authorization for 12- to 15-year-olds.
In a public health emergency the FDA can authorize unapproved products for temporary use. Its still available under emergency use authorization for children ages 12-15 and as a third dose for certain. Pfizer and BioNTech applied for full approval in May.
22 hours agoEmergency Use Authorization and not FDA approval was what many people have cited as their reason for not getting the COVID-19 vaccines. 1 day agoThe Food and Drug Administration has formally approved Pfizers COVID-19 vaccine. 23 hours agoThe US drug regulator on Monday granted full approval to the Pfizer IncBioNTech SE Covid-19 vaccine that had earned emergency-use authorization in December making it the first to secure such Food and Drug Administration validation as health authorities struggle to win over vaccine skeptics.
1 day agoThe Food and Drug Administration FDA on Monday granted full approval to Pfizers COVID-19 vaccine for individuals 16 years and older. The emergency use authorization. 21 hours agoThe FDAs full approval of the Pfizer vaccine is only for people ages 16 and up.
A survey from the Kaiser Family Foundation found 31 percent of people who are unvaccinated would get the shot if it moved from EUA to full approval. It filed for full approval called a biologics license application with the agency in early May. The FDAs approval of.
The Pfizer-BioNTech coronavirus vaccine received emergency approval Friday from the Food and Drug Administration FDA for use in people 16 years and older. The approval makes it. 4 hours agoBack in December 2020 the FDA allowed the Pfizer vaccine to be used under an emergency use authorization.
Normally doctors can prescribe FDA-approved products for other reasons than their original use. 1 day agoThe approval comes eight months after the FDA issued Pfizer its first emergency-use authorization for a Covid-19 vaccine on Dec. Now that it has gone through a full.
The Pfizer drug was the first in the country to be granted emergency use authorization from the FDA which it received in early December. Since then more than 204 million doses of the vaccine. On May 10 2021 the FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age.
1 day agoThe Food and Drug Administration on Monday granted full approval to Pfizer-BioNTechs coronavirus vaccine for people 16 and older making it the first to move beyond emergency-use. At least right now the FDA has not approved the COVID-19 vaccine and it is allowed to be distributed under an EUA emergency use authorization. 10 hours agoModerna has also applied for full approval and Johnson Johnson has said it hopes to apply later in the year.
15 hours agoAs Woodcock said the FDA previously approved the Pfizer vaccine for emergency use authorization EUA for people over the age of 12. 1 day agoThe vaccine also continues to be available under emergency use authorization EUA including for individuals 12 through 15 years of age and for the administration of a third dose in certain. The widely anticipated decision replaces the emergency use authorization granted by.
19 hours agoPfizer Moderna and Johnson Johnson vaccines were given emergency use authorization allowing them to be administered only during the public health emergency caused by the COVID-19 pandemic. Pfizers shot still is available for 12- to 15-year-olds under emergency use.

Is Emergency Use Authorization The Best Way To Get A Covid 19 Vaccine To The Public Bulletin Of The Atomic Scientists

Fact Check Coronavirus Vaccine Image Shared As Made In China Pfizer Covid 19 Vaccine Is A Hoax

Fda Vows All Hands On Deck Effort To Get Pfizer Coronavirus Vaccine Full Approval As Quickly As Possible The Washington Post

No comments