Cdc Pfizer Approval
CDC will continue to provide updates as we learn more. By Carolyn Crist December 14 2020 -- The CDC signed off on the Pfizer-BioNTech COVID-19 vaccine the agency announced on Sunday afternoon completing the final approval for people to.
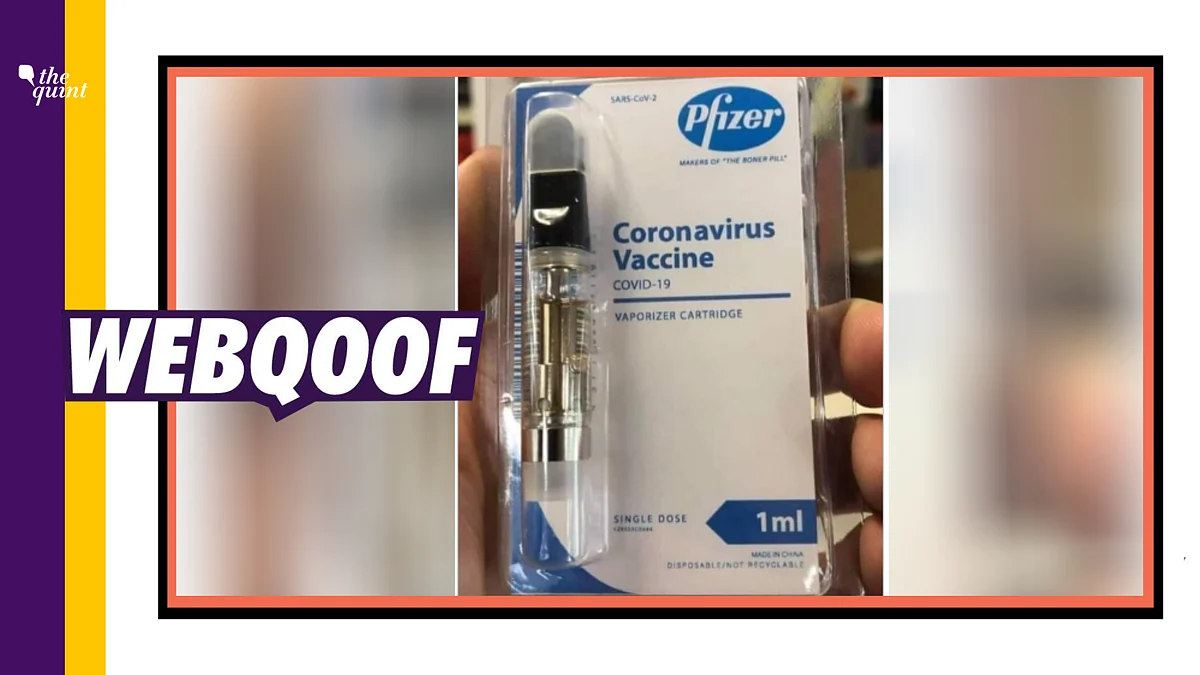
Fact Check Coronavirus Vaccine Image Shared As Made In China Pfizer Covid 19 Vaccine Is A Hoax
The Pfizer-BioNTech COVID-19 Vaccine Storage and Handling Summary pdf icon.
/cloudfront-us-east-1.images.arcpublishing.com/pmn/WI27KY4545GULLHIHLLH2IIJMI.jpg)
Cdc pfizer approval. 11 hours agoCDC chief on FDAs Pfizer approval. The Centers for Disease Control and Prevention gave final approval Friday to start administering Covid-19 booster shots to Pfizer and Moderna. 1 day agoToday the US.
A federal appeals court temporarily upheld the CDC. The following have been updated to reference the new storage and handling guidance from Pfizers Emergency Use Authorization EUA posted on May 19 2021. Food and Drug Administration expanded the emergency use authorization EUA for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of coronavirus disease 2019 COVID-19 caused.
CDC chief on FDAs Pfizer approval. In clinical trials the Pfizer-BioNTech vaccine was also highly effective at preventing laboratory-confirmed COVID-19 illness in adolescents aged 1215 years and the immune response in people aged 1215 years was at least as strong as the immune response in people aged 1625 years. Food and Drug Administration FDA approved vaccine to prevent COVID-19.
Regulators give full approval to Pfizers COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID. Hahn caved to the threats and approved the shot on December 11.
Food and Drug Administration Monday morning granted full approval to Pfizers COVID-19 two-shot vaccine. Updated information about the thermal shipper. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
There is no US. By Chris Menahan. Among those who have hesitated.
CDC does not recommend additional doses or booster shots for any other population at this time. This is a very powerful signal of safety More ABC News Linsey Davis speaks with Centers for Disease Control and Prevention Director Dr. In our news wrap Friday The Food and Drug Administration is ready to grant full approval to Pfizers COVID vaccine.
1 day agoPfizers two-dose COVID-19 vaccine is now fully approved by the US Food and Drug Administration making it the first shot against the coronavirus to make it. 18 hours agoThe US. COVID-19 Pfizer BioNTech Vaccine EUA Fact Sheet for Recipients.
Food and Drug Administration approved the first COVID-19 vaccine. The Pfizer-BioNTech COVID-19 Vaccine is a vaccine and may prevent you from getting COVID-19. This is a very powerful signal of safety In This Story.
The FDA only approved Pfizers experimental mRNA vaccine for emergency use last year after President Trumps chief of staff Mark Meadows told then-FDA commissioner Stephen Hahn to approve it by December 11th or be fired by the end of the day. 4 hours agoThe Pfizer-BioNTech COVID-19 Vaccine will now be marketed as Comirnaty for the prevention of COVID-19 in people older than 16 according to a. Rochelle Walensky about the Food and Drug Administrations full approval of the Pfizer vaccine.
Vaccine A vaccine is a biological preparation that provides active acquired immunity to a. The Centers for Disease Control and Prevention CDC cannot attest to the accuracy of a non-federal website. CDC recommends that people with moderately to severely compromised immune systems receive an additional dose of mRNA COVID-19 vaccine at least 28 days after a second dose of Pfizer-BioNTech COVID-19 vaccine or Moderna COVID-19 vaccine.
/cloudfront-us-east-1.images.arcpublishing.com/pmn/NZ33CZON6RFWHINHFHOXLIWSI4.jpg)
Cdc Releases New Guidelines For Vaccinated People Philly To Improve Vetting Of Vaccine Providers Pace Of Vaccinations Rising In Pa
/cloudfront-us-east-1.images.arcpublishing.com/pmn/WI27KY4545GULLHIHLLH2IIJMI.jpg)
Johnson Johnson Asks For Approval Of Its One Shot Vaccine Pa Lawmakers Beyond Frustrated With Vaccine Rollout Cases And Deaths Trending Down
/cloudfront-us-east-1.images.arcpublishing.com/pmn/PAZMMNKTJNE2RNQ7U6TXMU4ETQ.jpg)
Covid 19 Updates Case Numbers In Philadelphia Pa And N J Mandatory Vaccine For N J Teachers

Cdc Adviser Discuss Additional Coronavirus Doses For Vulnerable Patients The Washington Post
No comments